
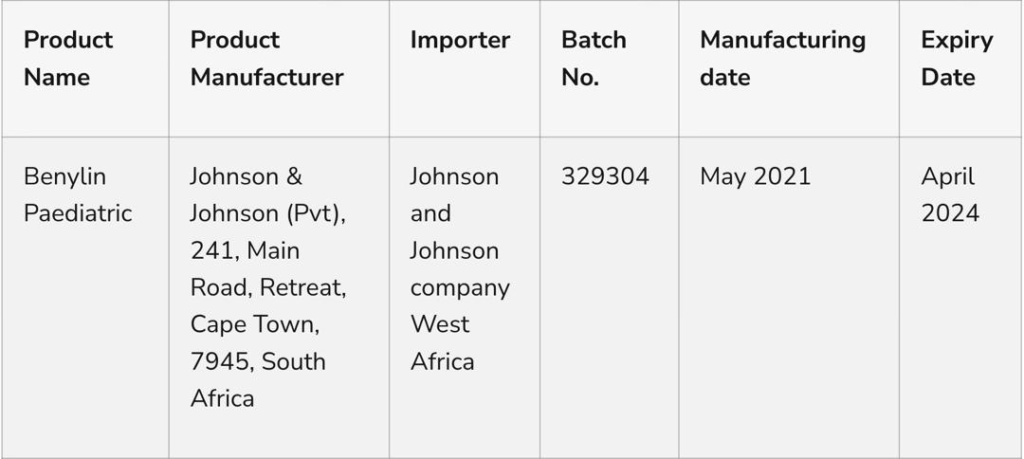
The National Agency for Food and Drug Control NAFDAC has alerted the public on the recall of one lot of Benylin Paediatric Syrup manufactured by Johnson & Johnson, following recent toxicity findings in the laboratory on the product.
A statement released by the agency today April 11 says laboratory analysis conducted on the product showed that it contains an unacceptable high level of Diethylene Glycol and was found to cause acute oral toxicity in laboratory animals.
Benylin Paediatric Syrup is indicated for the relief of cough and its congestive symptoms and for the treatment of hay fever and other allergic conditions in children aged 2 to 12 years.
Risk Statement
Diethylene Glycol is toxic to humans when consumed and can prove fatal. Toxic effects can include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state, and acute kidney injury which may lead to death.” the statement reads in part
NAFDAC implored importers, distributors, retailers, and consumers to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale, and use of substandard (contaminated) regulated products.
“Healthcare professionals and consumers are advised to report any suspicion of substandard and falsified medicines to the nearest NAFDAC office, call 0800-162-3322 or send an email to [email protected]” the agency said






Be First to Comment